Details of the Drug
General Information of Drug (ID: DMFOZ60)
| Drug Name |
aconitine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
aconitine; 302-27-2; CHEBI:2430; Acetylbenzoylaconine; NSC56464; 20-ethyl-3alpha,13,15alpha-trihydroxy-1alpha,6alpha,16beta-trimethoxy-4-(methoxymethyl)aconitane-8,14alpha-diyl 8-acetate 14-benzoate; 16-Ethyl-1alpha,6alpha,19beta-trimethoxy-4-(methoxymethyl)-aconitane-3alpha,8,10alpha,11,18alpha-pentol, 8-acetate 10-benzoate; NCGC00163146-01; CAS-302-27-2; DSSTox_RID_81535; DSSTox_CID_26319; SCHEMBL73323; DSSTox_GSID_46319; GTPL2617; DTXSID4046319; CHEMBL1979562; MolPort-002-527-310; Tox21_112015; NSC-56464; AKOS032428256
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
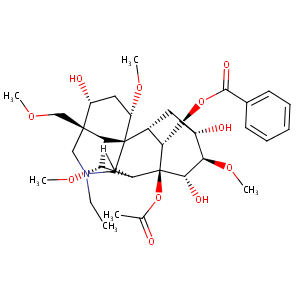 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 645.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
